Infrared Spectroscopy
Infrared Spectroscopy helps us to identify Organic molecules
· In IR (Infrared Spectroscopy), a beam of IR radiation passes through the sample.
· The IR energy is absorbed by the bonds in the molecules, increasing their vibrational energy
· Different bonds absorb different wavelengths.
The table below shows the frequencies different groups absorb ---
|
Functional group
|
Where it’s found |
Frequency/ Wavelength (cm^-1)
|
Type of absorption |
|
O-H |
alcohols |
3200 – 3750 |
strong, broad |
|
O-H |
carboxylic acids |
2500 – 3300 |
medium, very broad |
|
C-O |
alcohols, carboxylic acids & esters |
1100 – 1310 |
strong, sharp |
|
C=O
|
aldehydes, ketones, carboxylic acids & |
1680 - 1800 |
strong sharp |
O-H groups in alcohol and carboxylic acids tend to have broad absorptions due to their hydrogen bonding.
C=O groups in esters and carboxylic acids tend to have strong and sharp absorptions at around 1740cm^-1.
An example of the Ethanol Infrared Spectrum is shown below. Please have a look.
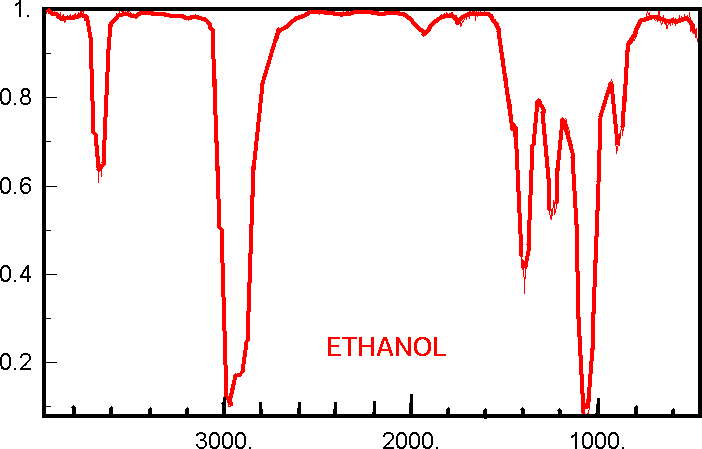
http://www.google.co.uk/imgresimgurl=http://wetche.cmbi.ru.nl/vwo/spplus/ir/spectra/ethanol3.gif&imgrefurl=http
://wetche.cmbi.ru.nl/vwo/spplus/ir/spectra/ethol.html&usg=__US46PPQYb0IEJettv43FO5uhZRE=&h=449&w=702&sz
=9&hl=en&start=70&zoom=1&tbnid=6XvGUHF_JD17aM:&tbnh=138&tbnw=215&ei=g4PCTdv0KoO28QPz1bHEBQ&prev=
/search%3Fq%3Dinfrared%2Bspectrum%2Bof%2Bethanol%26um%3D1%26hl%3Den%26safe%3Dactive%26sa%3DN26biw%3D1259%26bih3D83926tbm3Disch&um=1&itbs=1&iact=rc&dur=140&page=4&ndsp=23&ved=1t:429,r:15,s:70&tx=115&ty=55
Infrared Spectroscopy is a very useful. It is used in:
· breath analyser
· forensic analysis
· identification of polymer degradation etc.