NMR Spectroscopy
NMR provides information about an organic molecules structure
It can tell us:
· The different proton environments in the molecule (from the chemical shifts)
· The relative number of protons in each environment (from the relative peak area)
· The number of protons adjacent to a particular proton (from the splitting patterns.
The isotopes most commonly studied using NMR Spectroscopy are H1, C13, F19 & P31.
The reason NMR works is that nuclei with odd numbers of nucleons have a spin. Hydrogen nuclei are single protons, so they have spin.
The requirements for NMR spectroscopy are:
· A strong magnetic field applied using an electromagnet
· Low energy radio frequency radiation
Chemical shift is measured relative to Tetramethylsilane
Protons in different environments absorb energy of different frequencies. NMR measures the difference relative to the standard substance and this difference is Chemical sift. The standard substance is usually tetramethylsilane (TMS). Even though this molecule has 12 identical proton environments, it will only produce one peak which is given the chemical shift of 0. Therefore an NMR spectrum often shows this peak at 0 and is due to the TMS added to it for calibration purposes.
NMR tells you the Number of Environments and the Number of Protons in each
Every peak on the NMR spectrum is due to one or more protons in a specific environment. The area under the peak can tell us how many protons are present in that environment too.
For example: On the NMR Spectrum of ethanal, there are two peaks. So are two environments. The area ratio is 1:3, it means that there’s 1 proton in the environment at 9.6p.p.m. to every 3 protons in other environments.
Use a table to identify the proton causing the Chemical shift
|
Chemical shift (p.p.m.) |
Type of proton |
|
0.8-1.3 |
R-CH3 |
|
1.2-1.4 |
R-CH3-R |
|
2.0-2.9 |
R-COCH3 |
|
3.2-3.7 |
Halogen-CH3 |
|
3.6-3.8 |
R-CH2-Cl |
|
3.3-3.9 |
R-OCH2-R |
|
3.5-5.5 |
R-CH2OH |
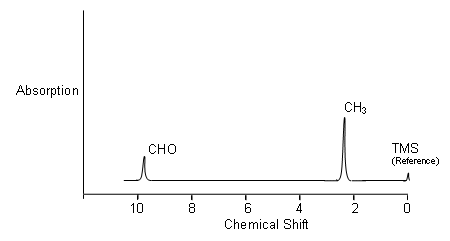
The example above shows that there is 1 hydrogen at 9.6p.p.m., so this must be due to an R-CHO group. The 3 hydrogen’s at 1.3p.p.m., means an R-CH3 group too.
Samples are dissolved insolvents without lone pairs
NMR analysis of a sample is usually carried out in solution. A solvent is required, but organic solvents contain carbon and hydrogen and these atoms themselves produce complex spectra. Therefore the sample should have no single protons. Deuterated solvents are often used as their hydrogen’s have been replaced by Deuterium. It is an isotope of hydrogen that it’s got two nucleons (neutron and proton), so there’s no spin on the nucleus. CCL4 is another solvent which can be used in NMR Spectrometry.
Spin-Spin Coupling Splits the peaks in an NMR Spectrum
In high resolution NMR spectra, the peaks are split into smaller peaks; it is due to the magnetic fields of neighbouring single protons interacting with each other. They always split into the number of neighbouring protons plus one-n+1 rule. For example, if there are three protons on the adjacent carbon, the peak will be split into 3+1=4. We can work out the number of neighbouring protons by looking at how many peaks they are split into:
· If a peak is split into two (a doublet) then there’s one neighbouring single proton
· If a peak is split into three (a triplet) then there’s two neighbouring single proton
· If a peak is split into four (a quartet) then there’s three neighbouring single proton
When the peals are split, it’s not as easy to see the ratio of the areas, so an integration trace is often shown. The height increases are proportional to the areas.
Labile Protons are identified using D²O
Protons attached either to Oxygen or Nitrogen is usually called labile protons because they can be rapidly exchanged
with neighbouring molecules. The chemical shift due to them is variable as they could be involved in hydrogen bonding. They make broad peak that isn’t split too.
Chemists identify labile protons by:
1) Running two spectrums of the molecule where one is with a little deuterium oxide in it.
2) If a labile proton is present, it will swap with deuterium and the peak therefore disappears due to the non-spinning characteristic if deuterium which has even number of nucleons.