Mass Spectrometry
Mass
Spectroscopy is used to find out Relative Molecular Mass (Mr)
Electrons in the spectrometer bombarded the sample molecules and break electrons off, forming ions. A mass spectrum is produced, showing the relative amounts of ions with different mass-to-charge ratios.
The molecular ions M+ gives the peak in the spectrum with the second highest mass-to-charge ratio, so it’s the last peak but one on the spectrum. This peak’s called the M peak. The M peak is the relative molecular mass if the molecule.
The Highest peak is called the base peak. Its relative abundance is 100%. It’s due to particularly stable fragment ions such as carbocations.
A spectrogram has a y axis which gives the abundance of ions, often as a percentage. The x-axis units are given as a ‘mass/charge’ ratio.
We can also use the M+1 Peak to work out the Number of Carbon Atoms in molecule.
The peak 1 unit to the right of the M peak is called the M+ 1 peak. It’s mostly due to the carbon-13 isotope which exists naturally. The equation to work out the number of Carbon atoms in a compound is:
Height of M+1 peak
------------------------ x100
Height of M peak
For example: If a molecule has a molecular peak with relative abundance of 32.17% and a M+1 peak with a relative abundance of 2.34%, how many carbon atom does it have?
Answer : (2.34/32.17) x100 = 7.27 Therefore the molecule contains 7 Carbon atoms.
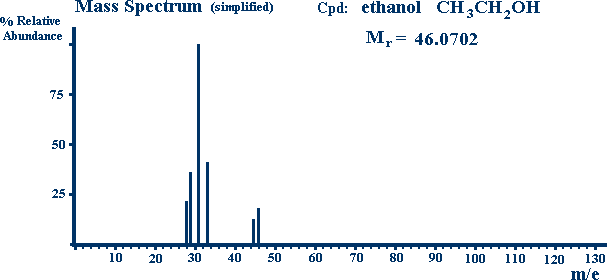
The Mass Spectrum of Ethanol is shown below.
gif&imgrefurl=http://toolboxes.flexiblelearning.net.au/demosites/series5/508/laboratory/studynotes/sntheinterpm
asspect.htm&usg=__Et6Ynu27Hcx4D40Vpfo7wvZpft4=&h=280&w=607&sz=4&hl=en&start=0&zoom=1&tbnid=ApOCIhlGm
WYBkM:&tbnh=78&tbnw=169&ei=8IPCTajhFMzs-gbavb3IAg&prev=/search%3Fq%3Dmass%2Bspectrum%2Bof%2Bethanol%26um%3D1%26hl%3Den%26safe%3Dactive%26biw%3D1259%26bih%3D839%26tbm%3Disch0%2C126&um=1&itbs=1&iact=hc&vpx=85&vpy=111&dur=343&hovh=152&hovw=331&tx=223&ty=102&page=1&ndsp=31&ve
d=1t:429,r:6,s:0&biw=1259&bih=839